Recent Blog Posts
We represent Litzy Banuelos a/k/a Emily Willis
We represent Litzy Banuelos a/k/a Emily Willis in her claims against Summit Malibu, view the article here.
Depo-Provera or Depo-SubQ Provera 104®
Attention: Depo-Provera or Depo-SubQ Provera 104® injections at least twice a week show an increased risk of cranial and spinal meningiomas. If you or a loved one developed meningiomas after receiving Depo-Provera or Depo-SubQ Provera injections and are in need of representation in filing a Depo-Provera lawsuit, call us James James Morris Law Firm… Read More »
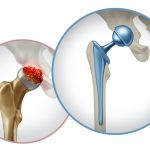
Important Metal on Metal Hip Update:
If you received a Metal on Metal Hip implant between 2005 and 2010 and are suffering from pain in the surgical hip, it may be due to increasing metal ions produced by the device as it wears over the years. The metal on metal implants can cause a condition called Adverse Local Tissue Reaction… Read More »
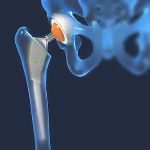
Types of Damages You Can Recover From a Hip Replacement Lawsuit
If you have suffered because of a defective metal-on-metal hip implant, you are entitled to compensation. An experienced hip replacement lawyer can help you file a lawsuit seeking the monetary payment you are owed. Read on to learn about the damages you may be able to collect in your defective hip implant lawsuit. If… Read More »
Types of Defective Hip Complications
Hip replacement devices–in particular, metal-on-metal (MOM) hip implants–carry a number of risks. Millions of hip implant devices have been recalled in recent years after it was discovered that the devices were defective as manufactured and designed. Defective hip implants carry a significantly higher risk of complications, causing illness and injury that can be painful,… Read More »

Where Can I Find Hip Replacement Lawyers?
If you have a metal-on-metal (MOM) hip implant built by DePuy, Biomet, Stryker, or other medical device manufacturers, you might have a claim for harm caused by the implant. A number of studies and court cases have determined that many industry-standard metal hip replacement devices were defective as designed and manufactured. Hundreds of thousands… Read More »
How to Choose an Attorney For Hip Replacement Suit
If you have or have ever had a metal-on-metal hip implant like the Depuy ASR, you may have suffered a number of unfortunate side effects. You have the right to seek compensation from the company that manufactured and sold the defective device. To build the strongest case with the best chance for maximum recovery,… Read More »
MoM Hip Replacement Can Lead to Heart Problems
We’ve discussed the risks of metal-on-metal (MoM) hip replacement devices a few times before. The devices are faulty and prone to becoming dislodged or broken. The various parts scrape together which, over time, can leak poisonous cobalt, chromium, and other metals into the bloodstream, causing a host of side effects. Metal poisoning can cause… Read More »
Cobalt Toxicity From Hip Replacement Can Cause Vision Damage
There was a time when medical device manufacturers saw metal-on-metal hip replacements as the next evolution of medical prosthetics. They touted the view that metal would last longer and remain more durable than the plastic products then being manufactured. This argument belied the underlying risks of metal-on-metal products. In recent years, evidence has come… Read More »

What Should You Do if You Suspect Your Metal Hip Replacement Is Defective?
We’ve talked about it before, but we’ll say it again: metal-on-metal hip replacement devices are dangerously defective. Tens of thousands of patients around the country have suffered pain, debilitation, and worse as a result of defective hip devices implanted over the last few decades. If you believe you’ve been fitted with a defective metal… Read More »